Background
ISO 13485 is the medical-device quality standard. It embeds risk-based planning, design, and process controls, validation (IQ/OQ/PQ), supplier oversight, traceability, and CAPA. The framework is widely recognized: the EU’s MDR/IVDR and many national regulators acknowledge or reference ISO 13485. For North America and the EU, 13485 is often a market-entry threshold. The standard spans the chain—design and development, component/raw-material supply, sterilization/clean processing, finished-device manufacturing, distribution, and technical services.

ISO 13485 certification
How Mastars Meets ISO 13485 Requirements
- Map risks and controls end-to-end; maintain DMR/DHR and change control.
- Qualify suppliers and materials; enforce incoming checks and traceability.
- Validate special processes (IQ/OQ/PQ); retain verification records.
- Monitor with SPC/MSA; calibrate CMM and gauges on schedule.
Control cleanliness and handling; train staff; close NC/CAPA loops.
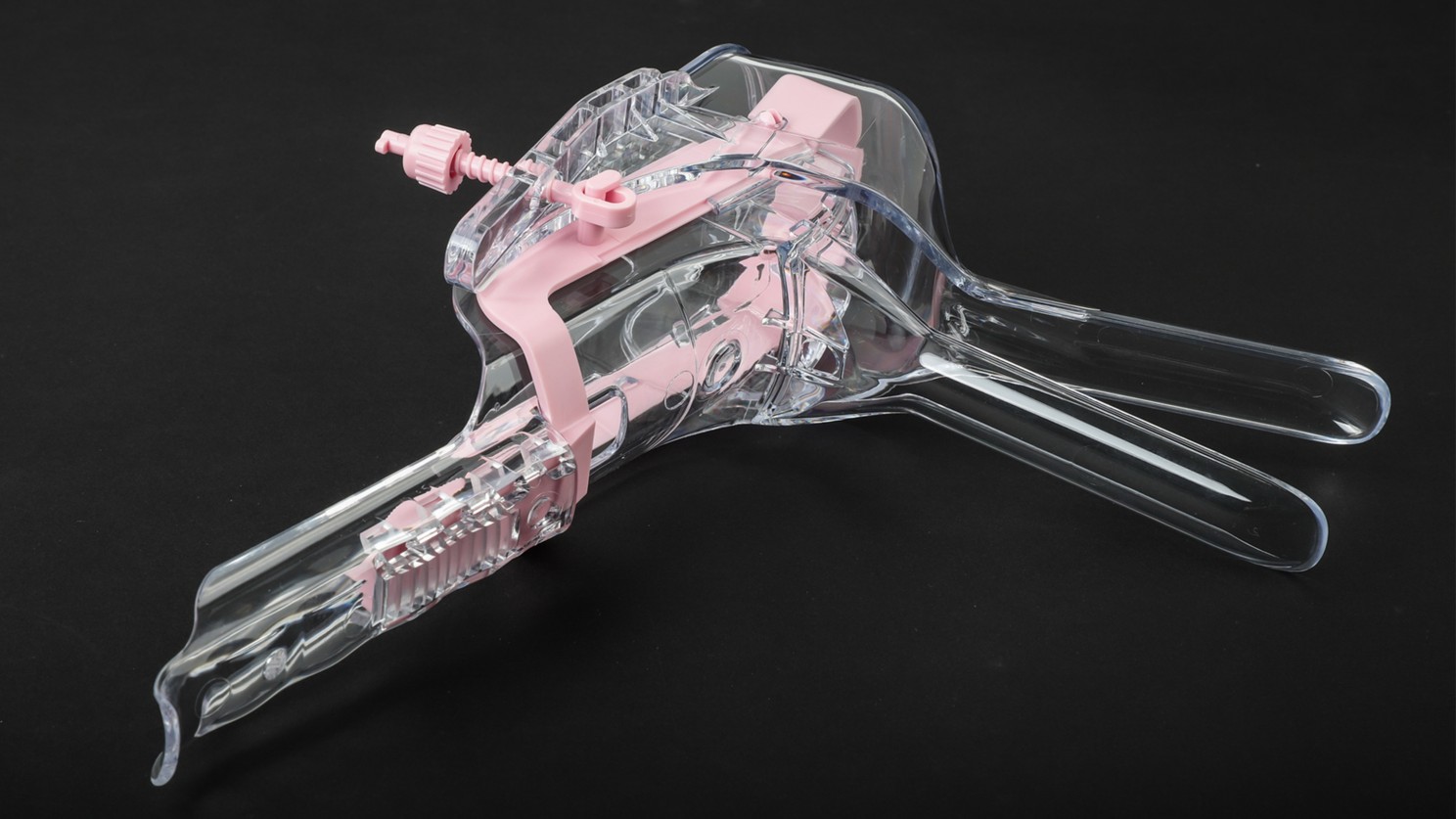
From a gynecological speculum client, showing ISO-compliant cleanroom assembly.
Faster, Safer Device Ramps With Certainty
✅ Rapid prototyping accelerates early design and testing.
✅ Lower compliance risk with audit-ready documentation.
✅ Stable quality from validated, monitored processes.
✅ Full lot and serial traceability across builds.
✅ One vendor for NPI, volume, and box-build.

From a breast pump client, Mastars has the ability for full box-build integration.
From Prototypes To Box-Build Applications
- Prototyping: CNC, vacuum casting, 3D printing, sheet metal.
- Molds & Plastics: moldmaking, injection molding, extrusion, silicone/LSR.
- Metals: die casting, CNC machining, MIM, extrusion.
- OEM/ODM: co-development, project management, NPI, assembly.
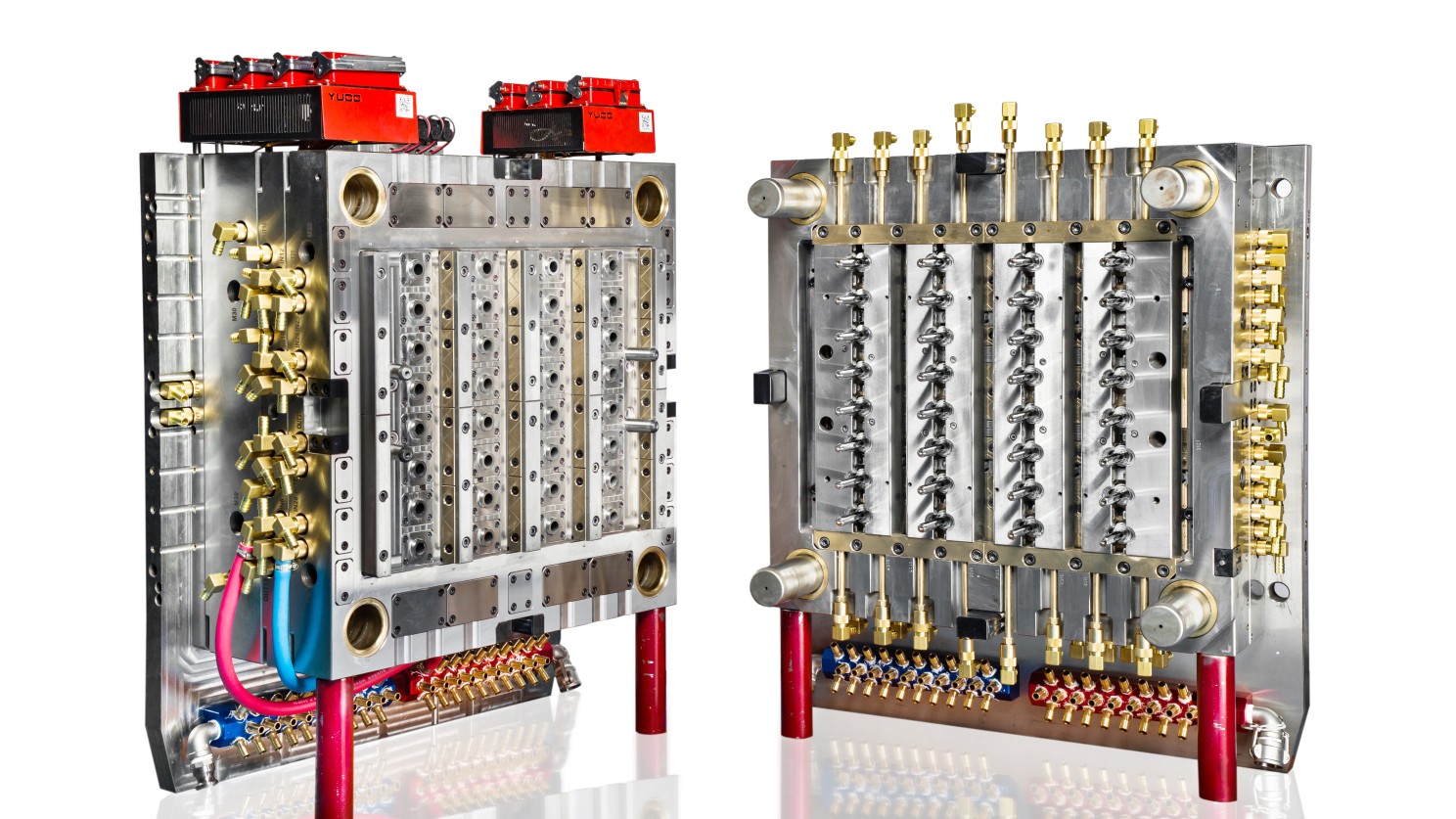
Virus tube injection mold. Mastars is capable of in-house mold design and machining.
One-stop Manufacturing System for Medical Device
Mastars pairs ISO 13485 controls with a one-stop manufacturing chain—giving small and mid-size medical teams faster prototyping starts and a complete path from raw material and component supply, through sterilization/clean processing and finished assemblies, to distribution and technical service, with stable quality and full traceability.

Clean packaging, sealing, and barcode traceability.
————————————————————————————————————————————————————————————————————————————————————————
Mastars, the world's most trusted one-stop small and medium-volume digital manufacturing service provider.
Hot Articles
Hot Tags
Manufacturing on Demand
Please fill in the following information to obtain plan details (information is confidential and not disclosed publicly), we will contact you within 24 hours, please keep your phone available!
Upload a 3D/2D model to see instant pricing, lead time, and DFM feedback.













