In the global healthcare industry, where patient safety, product reliability, and regulatory compliance are non-negotiable, medical molds serve as the unsung heroes of manufacturing. These specialized tools are the foundation for producing a vast array of medical devices and components—from disposable syringes and surgical instruments to complex implantable devices and diagnostic equipment. Unlike standard industrial molds, medical molds must meet extraordinarily strict standards for precision, biocompatibility, cleanliness, and traceability. This article delves into the critical role of medical molds, their key characteristics, manufacturing processes, applications, and the rigorous compliance framework that governs their production.
What Are Medical Molds?
Medical molds are custom-engineered tools used in injection molding, blow molding, or compression molding processes to fabricate medical devices and components from biocompatible materials. Their primary function is to replicate intricate designs with consistent accuracy, ensuring that every manufactured part meets the exact specifications required for medical use. Unlike consumer goods molds, which prioritize cost-efficiency and production speed, medical molds are designed first and foremost to uphold patient safety—this means eliminating risks such as material contamination, dimensional inconsistency, and surface defects that could compromise device performance or cause harm to patients.
The complexity of medical molds varies widely, from simple single-cavity molds for disposable items like pipettes to multi-cavity, hot-runner molds for high-volume production of precision components like catheter tips or implantable screws. Regardless of complexity, every medical mold must undergo rigorous testing and validation before it enters production, ensuring it adheres to global healthcare regulations and industry best practices.
Key Characteristics of High-Quality Medical Molds
The unique demands of the healthcare industry impose four non-negotiable characteristics on medical molds. These traits are the cornerstone of reliable, compliant medical device manufacturing:
1. Ultra-Precision Dimensional Accuracy
Medical devices often operate in critical anatomical spaces (e.g., blood vessels, joints, or the central nervous system) where even minor dimensional deviations can lead to catastrophic failures. Medical molds must achieve tolerances as tight as ±0.001 mm, ensuring every part produced is identical and fits or functions as intended. This level of precision requires advanced machining technologies—such as CNC milling, electrical discharge machining (EDM), and wire EDM—and strict process control during mold fabrication. For example, molds for insulin pens must maintain consistent wall thickness to ensure accurate drug delivery, while molds for surgical staples must produce uniform leg lengths to guarantee secure tissue closure.
2. Biocompatibility and Material Purity
Medical molds come into direct contact with raw materials that will eventually interact with the human body—either through implantation, ingestion, or skin contact. As such, mold materials (e.g., tool steel, stainless steel, or titanium) must be biocompatible, non-toxic, and resistant to corrosion or material leaching. Additionally, molds must be free of contaminants such as heavy metals, lubricants, or residual chemicals that could migrate into the final product. Reputable manufacturers use only FDA-approved or ISO 10993-certified mold materials and implement strict cleaning protocols (e.g., ultrasonic cleaning, plasma treatment) to eliminate contaminants.
3. Superior Surface Finish and Cleanability
The surface of a medical mold directly impacts the surface quality of the finished device. Rough or uneven mold surfaces can trap bacteria, harbor contaminants, or cause friction-related damage to delicate tissues (e.g., in catheters or endoscopes). Medical molds typically require a mirror-like surface finish (Ra ≤ 0.02 μm) achieved through precision polishing and buffing. This smooth surface not only ensures the device is easy to sterilize (a critical requirement for reusable medical tools) but also prevents material adhesion during the molding process, reducing waste and ensuring consistent part release.
4. Durability and Repeatability
Medical device production often requires high-volume runs (e.g., millions of syringes annually) or long-term production of implantable devices. Medical molds must be durable enough to withstand repeated cycles of heating, cooling, and pressure without degrading or losing precision. Mold materials are selected for their high hardness (e.g., HRC 50-60 for tool steel) and wear resistance, while advanced cooling systems (e.g., conformal cooling channels) are integrated to maintain consistent temperature and reduce cycle times. This durability ensures that the mold can produce tens of thousands—even millions—of parts with unwavering repeatability.
Manufacturing Process of Medical Molds: From Design to Validation
The production of medical molds is a highly controlled, multi-stage process that prioritizes quality, traceability, and compliance at every step. Below is a breakdown of the core stages:
1. Design and Engineering
The design phase is critical for ensuring the mold meets both technical specifications and regulatory requirements. Engineers collaborate with medical device designers to optimize the mold design for manufacturability (DFM), taking into account factors such as material flow, cooling efficiency, part ejection, and dimensional stability. Advanced CAD/CAM software (e.g., SolidWorks, Mastercam) is used to create 3D models of the mold, while simulation tools (e.g., Moldflow) predict potential issues like warpage, shrinkage, or air traps—allowing for adjustments before physical production begins. During this phase, designers also incorporate features to support traceability, such as serial numbers or QR codes on mold components.
2. Material Selection and Preparation
Mold materials are chosen based on the intended application, production volume, and material compatibility. Common materials include:
• Stainless Steel (e.g., 1.2083, 1.4404): Ideal for molds requiring corrosion resistance and easy sterilization, often used for disposable medical devices.
• Tool Steel (e.g., H13, S136): Offers high hardness and wear resistance, suitable for high-volume production of precision components.
• Titanium Alloys: Used for molds producing implantable devices, as titanium is biocompatible and resistant to bodily fluids.
All materials undergo rigorous incoming inspection, including chemical composition analysis and mechanical testing, to ensure compliance with industry standards (e.g., ISO 9001, ISO 13485).
3. Precision Machining
Medical mold components are machined using state-of-the-art equipment to achieve ultra-precise tolerances. Key machining processes include:
• CNC Milling: Used for shaping mold cavities and cores, with 5-axis CNC machines enabling complex geometries.
• EDM/Wire EDM: Ideal for creating intricate features (e.g., micro-holes, thin walls) that cannot be achieved with traditional milling, as it uses electrical discharges to remove material without physical contact—avoiding tool wear or part deformation.
• Grinding and Polishing: Ensures the mold surface meets the required finish, with automated polishing systems maintaining consistency across large batches.
Every machining step is documented, with process parameters (e.g., spindle speed, feed rate) recorded for traceability.
4. Assembly and Testing
Mold components (cavities, cores, runners, cooling systems, ejection systems) are assembled with extreme care, with each part aligned to within microns. Post-assembly, the mold undergoes a series of tests, including leak testing (for cooling channels), dimensional inspection (using CMMs), and surface finish verification (using profilometers). Any deviations are corrected before proceeding to the next stage.
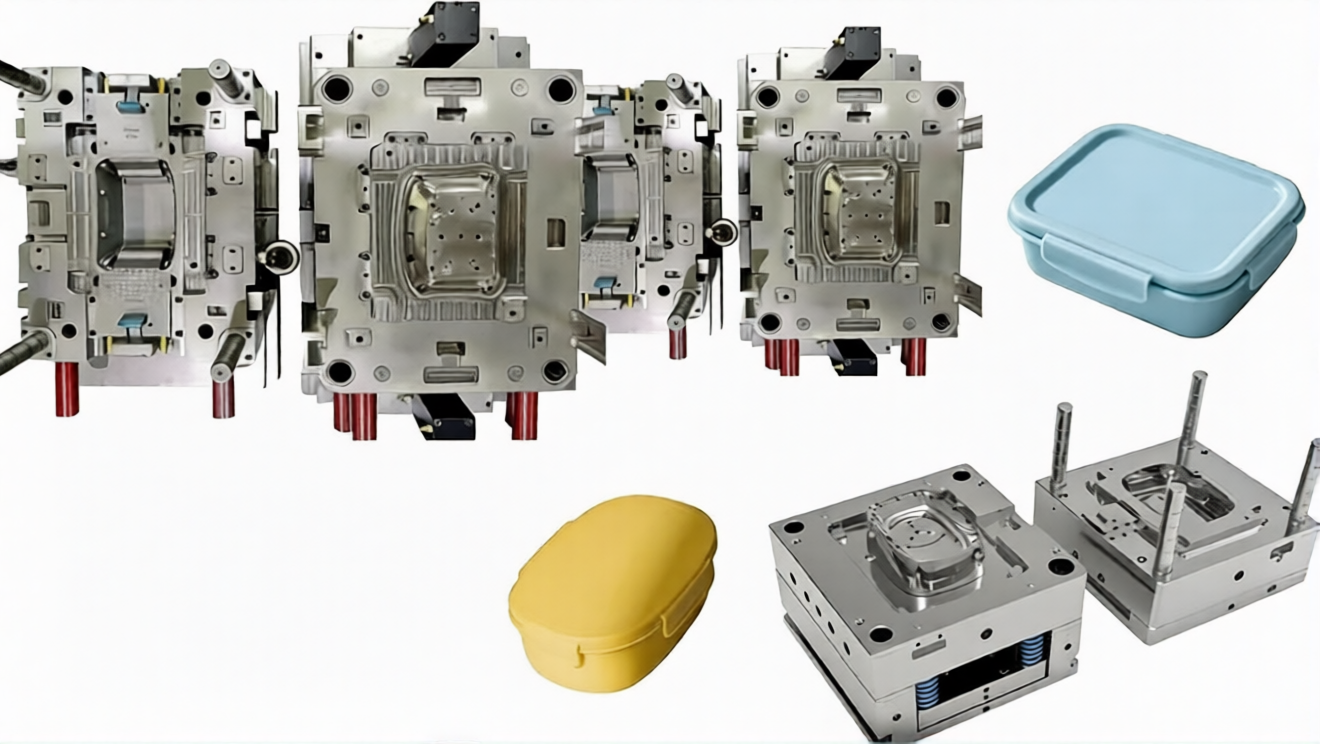
5. Validation and Qualification
Before a medical mold is approved for production, it must undergo rigorous validation to meet regulatory requirements (e.g., FDA 21 CFR Part 820). This includes:
• Process Validation: Running trial production runs to verify that the mold consistently produces parts meeting specifications.
• Cleanliness Validation: Testing the mold and finished parts for contaminants (e.g., particles, residual oils).
• Sterility Validation: For molds producing sterile devices, verifying that the mold can be effectively sterilized (e.g., via autoclaving or ethylene oxide) without degrading.
All validation data is documented in a comprehensive qualification report, which is required for regulatory submissions.
Critical Applications of Medical Molds
Medical molds are used to produce a diverse range of devices across the healthcare ecosystem, spanning diagnostics, treatment, surgery, and patient care. Key applications include:
1. Disposable Medical Devices
This is the largest application area for medical molds, encompassing products designed for single use to prevent cross-contamination. Examples include syringes, hypodermic needles, IV catheters, pipettes, test tubes, and surgical masks. Molds for these devices are typically multi-cavity (up to 128 cavities for syringes) to support high-volume production, with features like automatic part ejection to maximize efficiency.
2. Implantable Medical Devices
Implantable devices—such as pacemakers, artificial joints, spinal screws, and stents—require molds of the highest precision and biocompatibility. These molds must produce parts with zero defects, as any imperfection could lead to implant failure or adverse patient reactions. For example, molds for hip implants must maintain exact dimensional accuracy to ensure a proper fit with the patient’s bone, while molds for stents must create ultra-thin, uniform walls to withstand arterial pressure.
3. Diagnostic Equipment
Diagnostic tools like blood glucose monitors, pregnancy tests, and DNA testing kits rely on precision-molded components to ensure accurate results. Molds for these devices produce parts such as sensor housings, microfluidic channels (for lab-on-a-chip devices), and sample collection chambers—all of which require tight tolerances to maintain fluid flow or electrical conductivity.
4. Surgical Instruments
Reusable surgical instruments (e.g., forceps, scalpel handles, retractors) and their components are often produced using medical molds. These molds must create parts with durable, corrosion-resistant surfaces that can withstand repeated sterilization cycles. For example, molds for scalpel handles produce ergonomic designs with precise grip features, ensuring surgeons can operate with maximum control.
Regulatory Compliance: The Backbone of Medical Mold Manufacturing
The production of medical molds is governed by strict global regulations to ensure patient safety. Manufacturers must adhere to standards set by regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Organization for Standardization (ISO). Key regulatory requirements include:
• ISO 13485: A quality management system specifically for medical device manufacturers, which requires strict process control, traceability, and documentation throughout the mold lifecycle.
• FDA 21 CFR Part 820: Mandates current good manufacturing practices (cGMP) for medical devices, including mold design, production, validation, and maintenance.
• Biocompatibility Testing (ISO 10993): Ensures that mold materials and finished devices do not cause adverse biological reactions when in contact with the human body.
• Traceability: All mold components and production processes must be traceable, allowing manufacturers to recall products or identify issues in the event of a failure.
Non-compliance with these regulations can result in fines, product recalls, or even a ban on manufacturing—making regulatory expertise a critical competency for medical mold manufacturers.
The Future of Medical Molds: Innovation Driven by Healthcare Advances
As the healthcare industry evolves—with trends like personalized medicine, minimally invasive surgery, and digital health—medical molds are poised to become even more advanced. Key innovations shaping the future include:
• Additive Manufacturing (3D Printing): 3D printing is being used to produce complex mold components (e.g., conformal cooling channels) that cannot be manufactured with traditional methods. This technology also enables rapid prototyping of molds, reducing lead times for new medical devices.
• Smart Molds: Molds integrated with sensors to monitor temperature, pressure, and part quality in real time. This data-driven approach allows for proactive adjustments, reducing defects and improving production efficiency.
• Biodegradable Materials: As the industry shifts toward sustainability, molds are being designed to process biodegradable, biocompatible materials for disposable devices—reducing environmental impact without compromising patient safety.
Conclusion
Medical molds are more than just manufacturing tools—they are critical enablers of safe, effective healthcare. Their ability to produce precise, consistent, and compliant medical devices is essential for advancing patient care and driving innovation in the industry. As healthcare demands continue to grow, the role of medical mold manufacturers will only become more important—requiring a relentless focus on precision, quality, and regulatory compliance.
For medical device companies, partnering with a trusted medical mold manufacturer is not just a business decision—it is a commitment to patient safety. By prioritizing expertise, technology, and compliance, these manufacturers ensure that every mold produced meets the highest standards, laying the groundwork for life-saving medical devices that improve outcomes for patients around the world.Hot Articles
Manufacturing on Demand
Please fill in the following information to obtain plan details (information is confidential and not disclosed publicly), we will contact you within 24 hours, please keep your phone available!
Upload a 3D/2D model to see instant pricing, lead time, and DFM feedback.













